1 Which of the Following Represents Alpha Emission
We review their content and use your feedback to keep the quality high. Describe what changes occur during alpha decay.
Alpha decay tends to change the form of an isotope to another element.

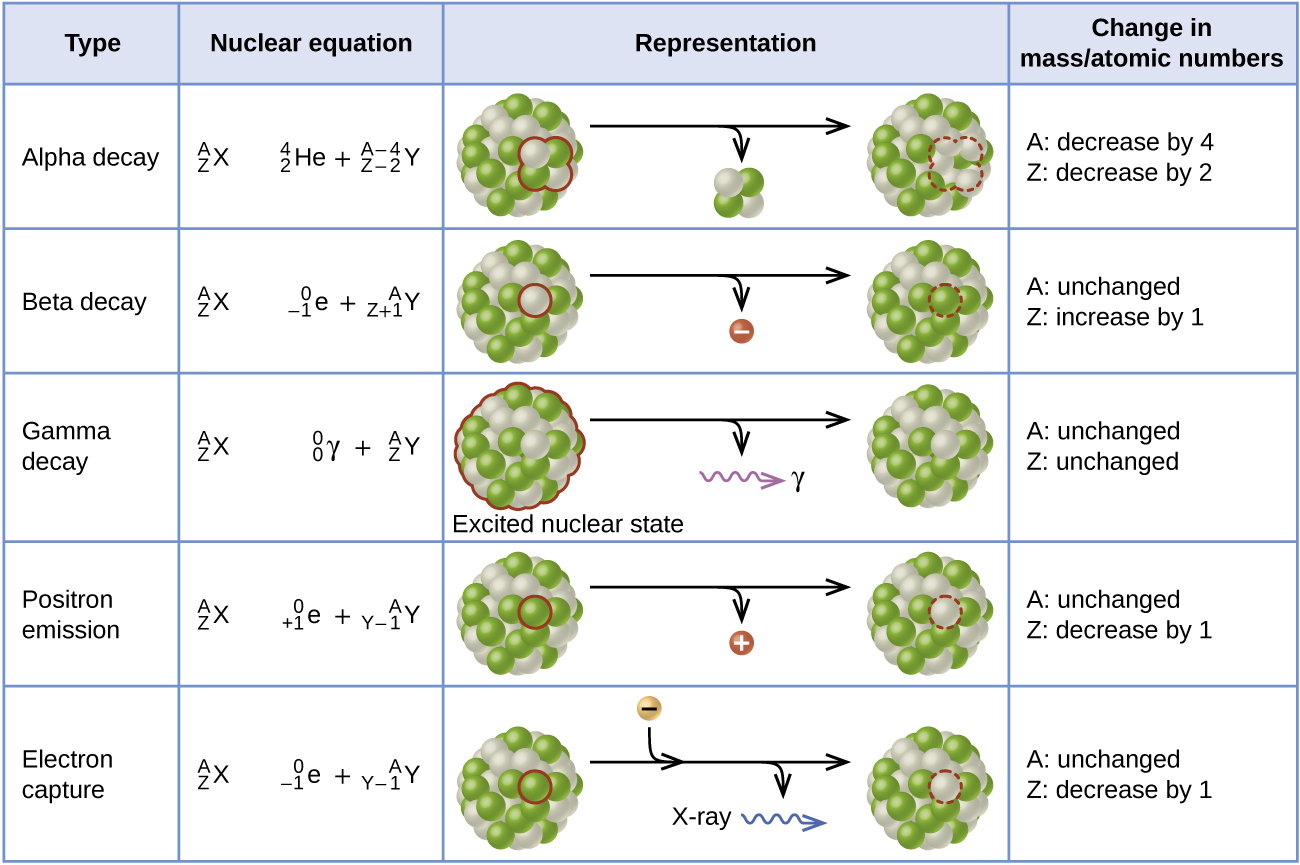
. 240 Pu 236U He 94 92 240 93 Np 249 Pu le 40 19K 18. If the nucleus of a radioactive element X of mass number A and atomic number Z emits an α particle a new element Y daughter nucleus is formed which has mass number equal to A-4 and atomic number equal to Z-2. Which of the following represents gamma emission.
Write balanced equations that represent the following nuclear reactions. Here Bi has atomic number as 83 and mass number as 189. The negative ion is the target atom.
C An electron has no charge and has negligible mass. What volume of helium at 250C and 120 atm would be obtained from a 250-g sample of Po-210 left to decay for 75 hours. This is alpha uranium decay and it implies the emission of the alfa particles and gamma emmission.
A Alpha emission by 155 70 Yb b Positron emission by Si 14 26 c Electron capture by 30 65 Zn d Electron emission by 100 41 Nb. Answer last option. B number of electrons.
A 222 218 4 86 84 2 Rn Po He B 220 2 86 82 4 Rn Pb He C 222 0 86 87-1 Rn Fr β D 4 226 86 2 88 Rn He Ra E 222 0 86 85 1 Rn At β 3. A nuclear equation that represents alpha emission is one that has He-4 as a product. Alpha decay is an emission of ₂⁴He nucleus.
Which of the arrows in the energy-level diagram represent transitions involving the greatest change in. We get 969 years. It is a highly energetic nuclear reaction.
2 question Which answer choice represents a balanced alpha emission nuclear equation. D A proton is positively charged and has a mass of 1 amu. The atomic nuclei emits an alpha particle when it undergoes alpha decay.
In the case of e there are 4 single electrons around. In case d there are 3 electron lone-pairs and 1 single electron around the Cl. The mass number and atomic number decreases.
The positive ion is electromagnetic. Polonium-210 decays to Pb-206 by alpha emission. Its half-life is 138 days.
Which one of the following equations correctly represents alpha decay of 222 86 Rn. In the case of c there are 13 single electrons around Cl. Since to get the K value use the half life equation t 12 0693K half-life is given here 288 years and putting the values and solving for time from 103 ppm to 1 ppm.
Which of the following nuclear equations correctly describes alpha emission. Two atoms must represent the same element if they both have the same. When undergoing an alpha emission formed daughter atom will has.
Which of the following nuclear reactions represents an electron or beta emission. Both a mass number and an atomic number. Complete the following equation of nuclear transmutation.
A 58 26 Fe B 58 28 Ni C. I hope this helps. Thus due to emission of an alpha particle atomic number Z decreases by two units and mass number decreases by 4 units.
The atomic emission spectra of a sodium atom on earth and of a sodium atom in the sun would be Emission lines are lines that are made when wavelengths of light are given off by cooling gases. Which of the arrows in the energy-level diagram represent absorption of energy and which represent emission of energy. This is a typical radioactive decay.
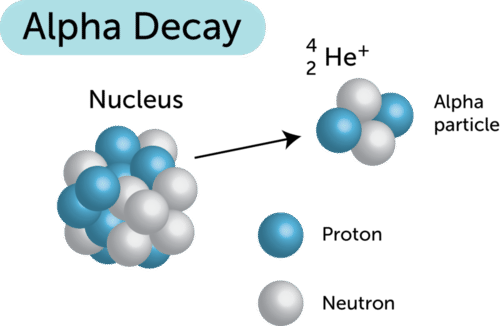
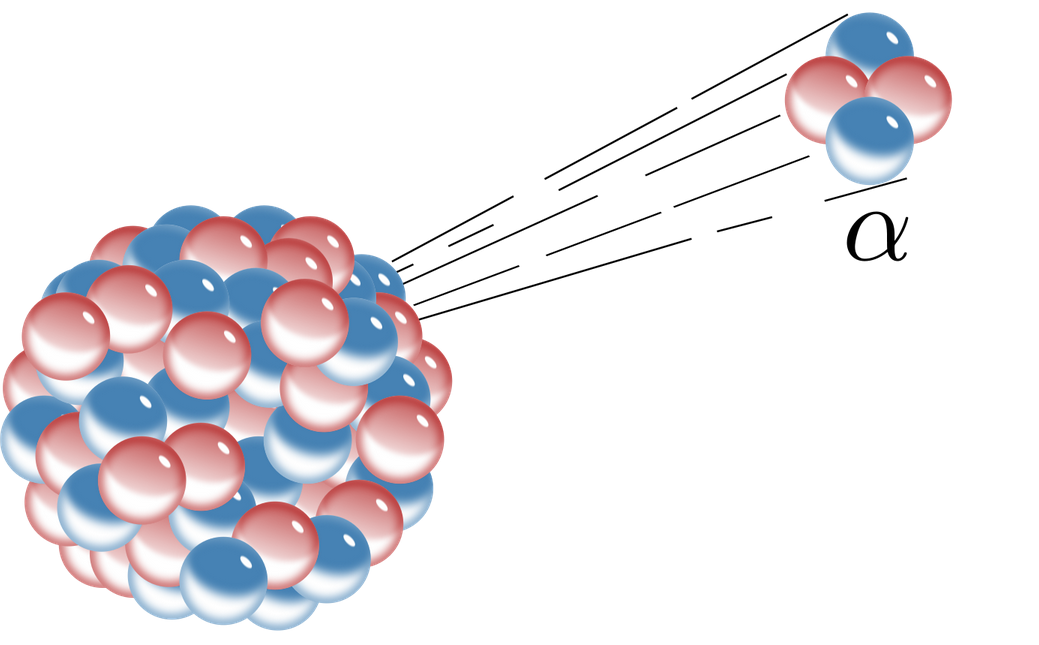
If an element undergoes an alpha decay the mass of the daughter nucleus formed is reduced by 4 compared to mass of parent atom while the atomic number of parent atom is reduced by 2. Ofen 898 2002. Up to 24 cash back Using the below symbols which of the following represents an alpha particle.
The emission of a positron whith the decrase of the atomic number. Alpha decay involves the emission of a high energy helium nucleus. Is the alpha particle represent gamma emission.
That said gamma emission is the result of the nucleus stabilizing itself from an excited state that was caused by some event such as an. In the case of b there are two electron lone-pairs and two single electrons around the Cl. Since it follows the 1st order kinetics Using the equation K 2303log aa-xtime.
The negative ion is the ion pair. Which one of the arrows in the energy-level diagram represents emission of light with the longest wavelength. After a radioactive nucleus has decayed via alpha emission the daughter nucleus has.
Which best describes a chain reaction associated with a nuclear reaction. The positive ion is the resulting atom. View the full answer.
When an atom undergoes alpha decay it emits 2 protons and two neutrons alpha particle. 60 28 Ni - 60 28 Niy D. 212 83 Bi - 212 84 Po0-1e C.
The negative ion is electromagnetic radiation. 027Fe Fe Oy 40K 40 18 Ar le 240 Pu 236 U He 94 92 Co le 57 26 240 93 240 Pu 94 Je O 57 Fe Fe Oy Question 2 1 point Which of the following nuclear reactions represents an a alpha emission. Experts are tested by Chegg as specialists in their subject area.
220 86 Rn - 216 84 Po4 2 He. Select the nuclide that completes the following nuclear reaction. Which of the following represents the correct nuclear equation for the beta emission of.
Therefore this equation represents the alpha decay of ²¹⁰₈₄Po. Alpha decay is a nuclear reaction that removes the nucleus from an alpha and helium particle with mass number 4. By this decay an unstable heavier atom tends to become a more stable atom.
In the case of a there are 4 electron lone-pairs around Cl. When ionization occurs which of the following is true. Who are the experts.
Science Chemistry QA Library Part 1. 222 222 222 222 58 0 27 1 Co β. B A neutron has a charge and has a negligible mass.
An atomic number decreased by four and a mass number decreased by two b. The following reaction represents what nuclear process. 228 89 Ac - 228 90 ThB B.

Fine Structure Constant Google Search Equations Golden Ratio Planck Constant

Ngc 6914 Is A Reflection Nebula Embedded Within The Diffuse Emission Nebulosity Of The Cygnus Region The Region Represents An Imm Nebula Celestial Bodies Ngc

Login To Your Benchprep Account Mcat Prep Mcat Medical School

Alpha Decay Definition Example Facts Britannica
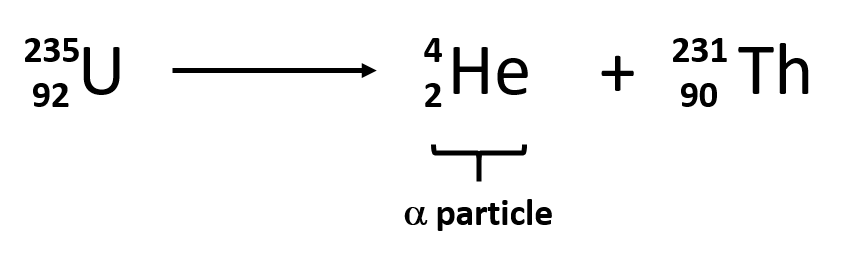
Ch103 Chapter 3 Radioactivity And Nuclear Chemistry Chemistry
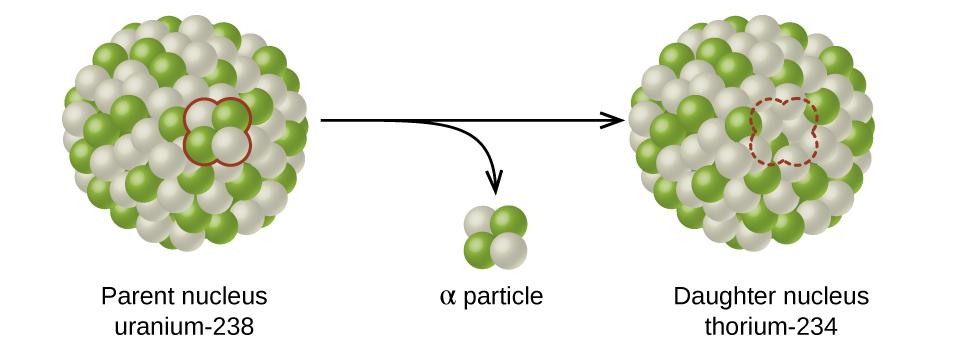
21 3 Radioactive Decay Chemistry

Alpha Centauri System Could Have Favorable Conditions For Life Information About Space University Of Colorado Boulder Space Science

Gm Jackson Physics And Mathematics How To Derive The Laplace Operator Laplacian For Cartesian Coordinates Laplace Physics And Mathematics
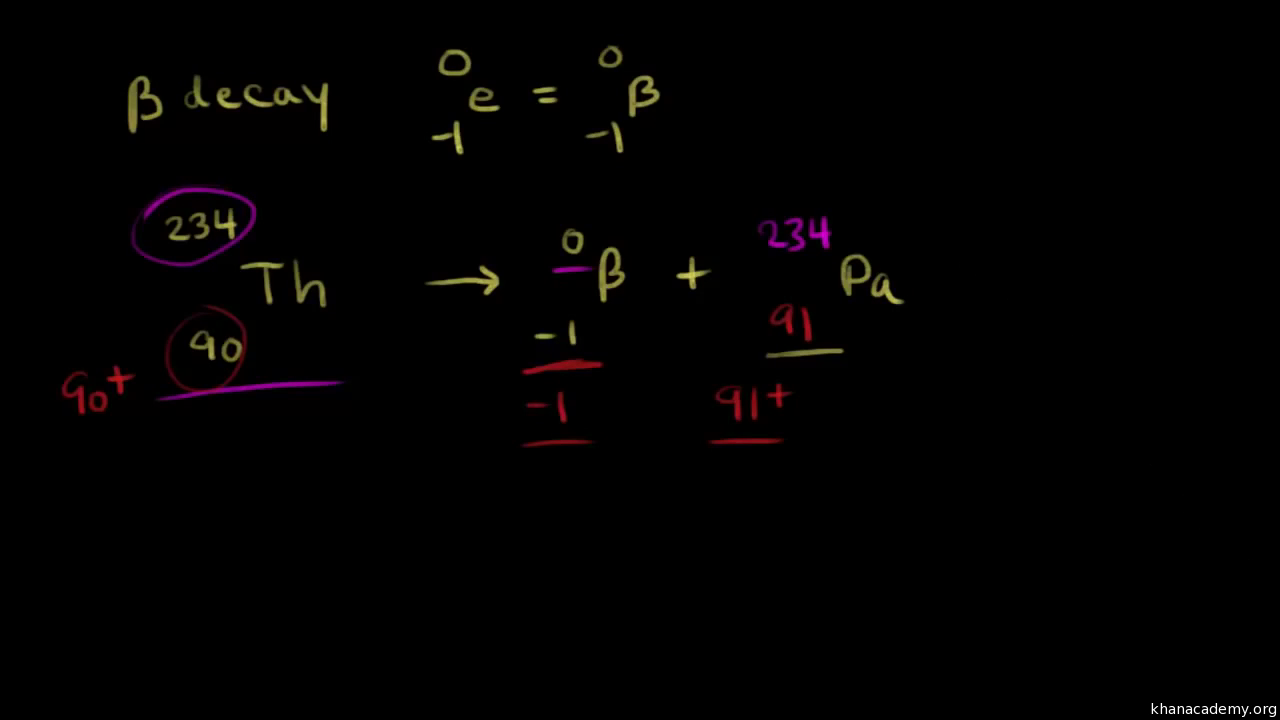
Writing Nuclear Equations For Alpha Beta And Gamma Decay Video Khan Academy

Pin By Delights On Homeschool Science Nuclear Reaction Equations Nuclear

Today In Chemistry History Ernest Rutherford S Birthday Ernest Rutherford Chemistry Education Chemistry Lessons

Alpha Decay Beta Decay Gamma Decay Electron Capture Positron Production Nuclear Chemistry Youtube

Bold Vivid Painted Bag Part Ii Painted Bags Drawing Bag Bags
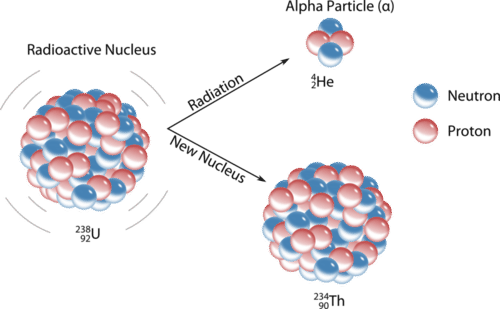
11 4 Nuclear Decay Chemistry Libretexts
11 2 Nuclear Equations Chemistry Libretexts

Alpha Decay Beta Decay Gamma Decay Electron Capture Positron Production Nuclear Chemistry Youtube

Kubota X Tractor A Completely Autonomous Tractor That Represents The Future Of Farming Public Transportation Design Transportation Design Kubota

Comments
Post a Comment